Davidson Lab Gene Regulatory Networks: Difference between revisions
imported>BArsh Created page with " Davidson Lab Gene Regulatory Networks <div align="left"> Contents <blockquote>'''ENDOMESODERM GENE NETWORK''' #BioTapestryViewer|BioTapestry Interactive Network Viewe..." |
imported>BArsh No edit summary |
||
| Line 51: | Line 51: | ||
<div align="left"> | <div align="left"> | ||
BioTapestry Interactive Network Viewer | == BioTapestry Interactive Network Viewer == | ||
<blockquote>The endomesoderm network model can be viewed using the [http://www.biotapestry.org/ '''BioTapestry Interactive Network Viewer'''], which is now a web application that runs in all modern browsers. BioTapestry was originally developed by William Longabaugh, Eric Davidson, and Hamid Bolouri, supported by a grant by NIH General Medical. Active development is continuing with support from NIH Child Health and Human Development. The most recent release is [http://www.biotapestry.org/#whatsNew Version 7] (September 2014), which supports both the new web browser-based Viewer, and the traditional Java Editor. The static-image version and Java WebStart Viewer version of the endomesoderm network have been discontinued. | <blockquote>The endomesoderm network model can be viewed using the [http://www.biotapestry.org/ '''BioTapestry Interactive Network Viewer'''], which is now a web application that runs in all modern browsers. BioTapestry was originally developed by William Longabaugh, Eric Davidson, and Hamid Bolouri, supported by a grant by NIH General Medical. Active development is continuing with support from NIH Child Health and Human Development. The most recent release is [http://www.biotapestry.org/#whatsNew Version 7] (September 2014), which supports both the new web browser-based Viewer, and the traditional Java Editor. The static-image version and Java WebStart Viewer version of the endomesoderm network have been discontinued. | ||
| Line 57: | Line 58: | ||
[http://grns.biotapestry.org/SpEndomes/ Click Here to View the Endomesoderm Model Using the BioTapestry Interactive Network Viewer] | [http://grns.biotapestry.org/SpEndomes/ Click Here to View the Endomesoderm Model Using the BioTapestry Interactive Network Viewer] | ||
</blockquote> | </blockquote> | ||
About The BioTapestry Editor | |||
== About The BioTapestry Editor == | |||
<blockquote>BioTapestry is an interactive tool for building, visualizing, and simulating genetic regulatory networks. The network '''viewer''' used here is a simplified, browser-based version of the full-featured network '''editor''', which is available from [http://www.biotapestry.org BioTapestry.org]. | <blockquote>BioTapestry is an interactive tool for building, visualizing, and simulating genetic regulatory networks. The network '''viewer''' used here is a simplified, browser-based version of the full-featured network '''editor''', which is available from [http://www.biotapestry.org BioTapestry.org]. | ||
| Line 63: | Line 66: | ||
* BioTapestry development is ongoing, with continuing new releases that improve the functionality and ease-of-use of the software. More information on plans for future development are available on the [http://www.biotapestry.org BioTapestry home page]. | * BioTapestry development is ongoing, with continuing new releases that improve the functionality and ease-of-use of the software. More information on plans for future development are available on the [http://www.biotapestry.org BioTapestry home page]. | ||
</blockquote> | </blockquote> | ||
BioTapestry Viewer "Quick Start" User's Guide | |||
== BioTapestry Viewer "Quick Start" User's Guide == | |||
<blockquote>'''Levels of Network Models''' [[File:TreeView.png|362x400px|Tree View]] | <blockquote>'''Levels of Network Models''' [[File:TreeView.png|362x400px|Tree View]] | ||
Revision as of 22:52, 26 March 2020
Davidson Lab Gene Regulatory Networks
Contents
ENDOMESODERM GENE NETWORK
BioTapestry Interactive Network Viewer
BioTapestry Viewer "Quick Start" User's Guide
- Endomesoderm Specification 21 to 30 Hours: View From All Nuclei
- PMC Specification 6 to 30 Hours: View From All Nuclei
- Endomesoderm Specification 6 to 18 Hours: View From All Nuclei
ECTODERM GENE NETWORK
- BioTapestry Interactive Network Viewer For Ectoderm
- Ectoderm Network Views
- Mesomere Lineages Network 6 to 17 Hours: View From All Nuclei
- Oral Ectoderm Network 18 to 30 Hours: View From All Nuclei
- Lateral and Aboral Animal Ectoderm 18 to 30 Hours: View From All Nuclei
- Veg1 Ectoderm 21 to 30 Hours: View From All Nuclei
- Ectoderm QPCR and Nanostring Data Tables
HIGH DENSITY TIMECOURSE DATA
ENDOMESODERM GENE NETWORK
BioTapestry Interactive Network Viewer
The endomesoderm network model can be viewed using the BioTapestry Interactive Network Viewer, which is now a web application that runs in all modern browsers. BioTapestry was originally developed by William Longabaugh, Eric Davidson, and Hamid Bolouri, supported by a grant by NIH General Medical. Active development is continuing with support from NIH Child Health and Human Development. The most recent release is Version 7 (September 2014), which supports both the new web browser-based Viewer, and the traditional Java Editor. The static-image version and Java WebStart Viewer version of the endomesoderm network have been discontinued.
Click Here to View the Endomesoderm Model Using the BioTapestry Interactive Network Viewer
About The BioTapestry Editor
BioTapestry is an interactive tool for building, visualizing, and simulating genetic regulatory networks. The network viewer used here is a simplified, browser-based version of the full-featured network editor, which is available from BioTapestry.org.
- With the current version of BioTapestry, the user can: 1) draw networks by hand, or 2) specify a list of interactions either in interactive tables or comma-separated value (CSV) input files and then have the network laid out automatically. Also, when provided with separate tables of experimental data, BioTapestry can automatically show hourly changes in the network state.
- BioTapestry development is ongoing, with continuing new releases that improve the functionality and ease-of-use of the software. More information on plans for future development are available on the BioTapestry home page.
BioTapestry Viewer "Quick Start" User's Guide
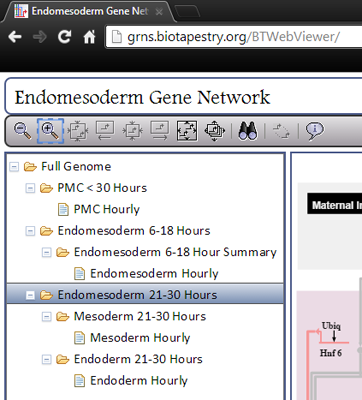
BioTapestry organizes the network model into four levels that are outlined in a tree view on the left side of the window. You can choose the view you want by clicking on it in this tree view. The four levels are:
Level 1: The Full Genome. This top level view shows the View from the Genome (VfG). This view shows only one instance of every gene, and all its inputs and outputs, regardless of when or where they occur. For example, the Tgif gene in this view show inputs that are present in both the PMCs and Veg2 Endoderm.
Level 2: This level shows four different views of the developing embryo. The first is a summarizing overview, that combines some features from the other three views. This is view you see when you start the viewer. Each of the other three views is called a View from All Nuclei (VfA). The three VfAs are for the PMC region up to 30 hours, the Endomesoderm from 6 to 18 hours, and the Endomesoderm from 21 to 30 hours. Note that endomesoderm development is depicted in two separate VfAs because of the increasing complexity of the separate territories over time, while PMC development can be represented in a single VfA.
Level 3: The third level can be thought of as a summation of all the hourly Views from the Nucleus (VfNs) that are located beneath it in the hierarchy. For example, the Mesoderm 21-30 Hours view is the superposition of the 10 Mesoderm Hourly VfNs below it. Note, however, that the PMC branch omits this level.
Level 4: The bottom level shows the View from the Nucleus (VfN), which depicts a a particular region at a particular hour. You can select the hour using the time slider, which is described below.
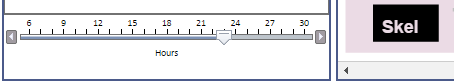
Whenever an "hourly" view is selected (the lowest level in the hierarchy), the viewer displays a time slider in the lower left corner that allows you to select the hour you wish to view by dragging the slider. Clicking to the left or right of the slider also moves it, and the left and right keyboard arrows are active (allowing fast hour switching) if you first click on the slider with the mouse.
The BioTapestry Viewer toolbar provides several important functions. As shown in the picture at left, there are buttons for (going left to right) zooming out one step, zooming in one step, zooming to all selected items (only active if something is selected), cycling backwards through the selected items (only active if multiple items are selected), zooming to show the current selected item (only active if multiple items are selected), cycling forwards through the selected items (only active if multiple items are selected), zooming to view the current model, zooming to bound all the models in the hierarchy, searching for genes/nodes, clearing current selections (only active if something is selected), and viewing the about dialog.
To select a gene, node, or link segment, just click on it. For genes, make sure to click in the areas highlighted in orange shown in the picture at left: either the gene name or the gene itself. To select more than one thing, hold down the Shift key while clicking on each new item. Use the toolbar button to clear all current selections.
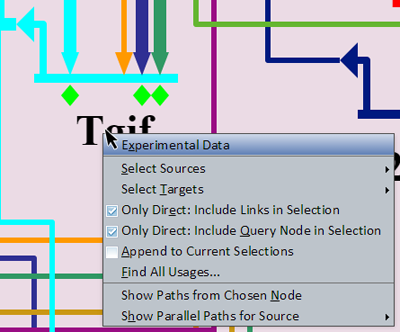
Right-clicking on a gene brings up a menu of options for that gene (see picture at left). (Note to Mac users: If you're using a one-button mouse, press the Control key while clicking the mouse to do this.) Make sure to click in the areas highlighted in orange shown in the picture in the previous section: either the gene name or the gene itself. (If you miss these areas, you will instead activate the menu for the underlying region or an inbound link.) Also, trying to select genes that are grayed out with no active inputs or outputs will not bring up this menu. Possible operations are:
- Experimental Data: This will pop up a window that shows the associated QPCR data, time expression profile data, and temporal input information for the gene.
- Select Sources: This is used to select the sources that have inputs into this gene, and has two sub-options, Select Only Direct Sources (will not pass through other nodes), and Pass Thru Nodes to Select Gene Sources (will pass through other nodes to reach source genes).
- Select Targets: This is used to select the targets of this gene, and has two sub-options, Select Only Direct Targets (will not pass through other nodes), and Pass Thru Nodes to Select Gene Targets (will pass through other nodes to reach source targets).
- Only Direct: Include Links in Selection: For the 'Select Only Direct' sub-option of Select Sources and Select Targets, checking this box will cause any links between the source or target to be selected as well. This is an application-wide setting.
- Only Direct: Include Query Node in Selection: For the 'Select Only Direct' sub-option of Select Sources and Select Targets, checking this box will cause the query node (node which opened this popup) of the source or target to be selected as well. This is an application-wide setting.
- Append to Current Selections: Checking this box will cause any new selections which result from a command to be appended to current selections rather than replacing them. This is an application-wide setting.
- Find All Usages...: This will pop up a dialog that lists all the other places where the gene appears. Select a list entry and then click on the Go to Selected Node button to zoom to the other places the gene appears.
- Show Paths from Chosen Node: Enables a click-selection mode (turning the cursor into a crosshairs) allowing the selection of a node from which to find any indirect paths to the source node, should they exist. A display (new window if allowed, otherwise a dialog in the current browser window) will open listing the possible paths.
- Show Parallel Paths for Source: A submenu allows you to choose from the list of all inputs into the gene. Choosing one of these inputs will bring up a display that shows alternative indirect paths from the source to the target.
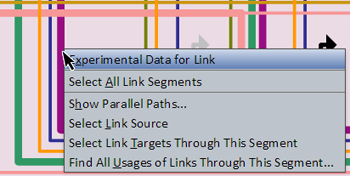
Right-clicking on a link segment brings up a menu of options for that link (see picture at left). (Note to Mac users: If you're using a one-button mouse, press the Control key while clicking the mouse to do this.) Possible operations are:
- Experimental Data for Link: This will pop up a window that shows the associated QPCR data, time expression profile data, and temporal input information for the link. If more than one link passes through this segment, a dialog with a drop-down allowing for selection of a specific link segment will open first.
- Select All Link Segments: This will select the entire tree of links for the link source. You can then use the Zoom to Selection toolbar button to see the whole tree in the view.
- Show Parallel Paths...: This works like the Show Parallel Paths for Source option for genes (see above). It is only active if you click on a link segment that does not branch before reaching its single target.
- Select Link Source: This will select and zoom to the source of the link.
- Select Link Targets Through This Segment: This will select and zoom to the targets of the links that are downstream of the clicked-on segment (this is usually not the same as all the targets of the link source).
- Find All Usages of Links Through This Segment...: This is very much like the Find All Usages... option for genes (see above), except it gives a list of links that are passing through the clicked-on link segment.
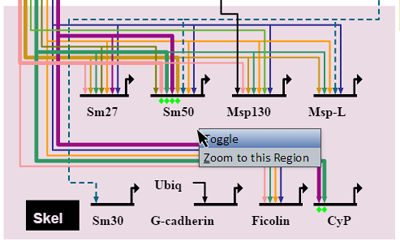
Right-clicking on a colored region brings up a menu of options for that region (see picture at left). (Note to Mac users: If you're using a one-button mouse, press the Control key while clicking the mouse to do this.) Possible operations are:
- Toggle: Toggles the region to hide or show region contents.
- Zoom to this Region: Zooms the view to bound the region.
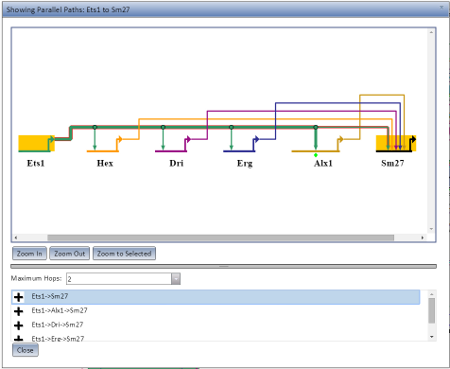
The Pathing Display opens when you choose Show Parallel Paths from Source or Show Parallel Paths from Chosen Node from the Gene Information pop-up. It will preferentially open as a new browser tab or window if it can (depending on your browser settings), but if not it will open as a dialog in the current browser window. An example of the Pathing Display open as a dialog is visible at the left.
The pathing model displayed will show all nodes along the possible route, with the current route highlighted in selection colors. There are three zoom options (Zoom In, Zoom Out, and Zoom to Selected) which behave the same as in the main application display. The maximum number of hops to allow for a potential route can be set using the Maximum Hops drop-down. A given rooute can be highlighted by selecting it from the list below the Maximum Hops limit.
Footnotes describing features of the network diagram are shown as red numbers. If you move your mouse over the footnote, the associated text will be displayed in the box at the bottom of the BioTapestry window (see picture at left). Clicking on the note will keep the text displayed even if you move your mouse elsewhere.
[[|]] Endomesoderm Network Views Back to Top
The increasing complexity of the network model means that a single overview network is no longer being provided. However, the view below of the endomesoderm territories after 21 hours is the closest to the traditional single overview network diagram, and the two network diagrams following it present more complete information for earlier time periods. All three "View from All Nuclei" (VfA) diagrams purport to illustrate the sum of linkages that are functional in the different places and the different stages of the endomesodermal specification process, including the Endomesoderm Specification 21 to 30 Hours, PMC Specification 6 to 30 Hours, and Endomesoderm Specification 6 to 18 Hours.i
|
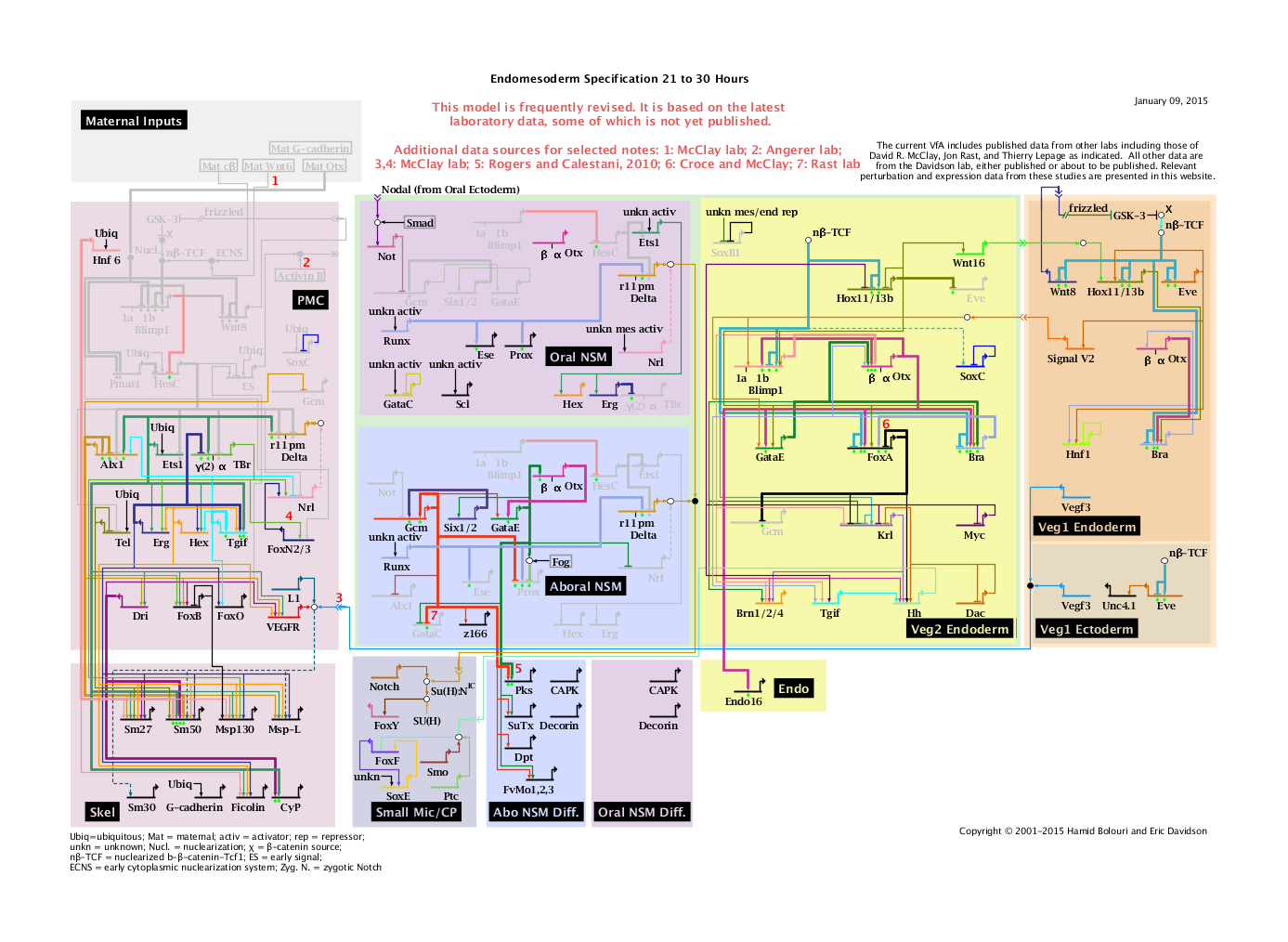
'''Endomesoderm Specification 21 to 30 Hours: View From All Nuclei''' Back to Top |
Notes
1: Data from McClay Lab.
2: Data from Angerer Lab.
3: Signal and Sm30 activation only after PMC ingression. Data from McClay; extrapolated from Lytechinus variegatus.
4: TBr->FoxN2/3 data from McClay; extrapolated from Lytechinus variegatus.
5: Gcm->Pks and GataE->Pks data from Rogers and Calestani, 2010.
6: Bra->FoxA data from WMISH in Bra MASO by Croce and McClay: same result from EHD lab.
7: Data from Rast Lab.
The architecture of the network is based on perturbation and expression data, on data from cis-regulatory analyses for several genes, and on other experiments discussed in text. See the endomesoderm network perturbation QPCR data for quantitative results of perturbation experiments and temporal details. Each short horizontal line from which a bent arrow extends to indicate transcription represents the cis-regulatory element that is responsible for expression of the gene named in the domain shown. Embryonic gene expression was perturbed in specific ways by use of antisense morpholino-substituted oligonucleotides by introduction of mRNA encoding Engrailed repressor domain fusions or mRNA encoding the normal protein product of the gene; by introduction of genetic expression constructs; by interference with the β-catenin/Tcf signal transduction system using overexpression of mRNA encoding an intracellular domain of cadherin; or by interference with the Notch (N) signaling system by overexpression of mRNA encoding the extracellular domain of the N receptor. RNA was extracted from embryos grown from eggs injected with the respective perturbation reagents and converted to cDNA. This was utilized in multiplexed quantitative PCR (QPCR) experiments to assess effects of each perturbation on the other genes indicated in the Figure. The arrows and barred lines indicate the normal function of the gene (activation or repression) as deduced from changes in transcript levels due to the perturbations. Minimum significance levels for inclusion in the model are three-fold increases or decreases in amount of transcript as a result of the perturbation; effects range up to several hundred fold. The relationships shown may in some cases be indirect, though all known or suspected indirect relationships have been excluded from the model. For linkages that are direct each input arrow constitutes a prediction of specific transcription factor target site sequence(s) in the relevant cis-regulatory control element. Experimental cis-regulatory results provide additional direct evidence; green diamonds indicate reported experimental evidence validates expected target site. Many genes are initially expressed over broader ranges, and their expression later resolves to the definitive domains indicated by their backgrounds. The rectangles in the lower tier of the diagram (with the exception of Small Mic/CP) show downstream differentiation genes. Dashed lines indicate inferred or possibly indirect relationships. Arrows inserted in arrow tails indicate intercellular signaling interactions. Large open ovals represent cytoplasmic biochemical interactions at the protein level, e.g., those responsible for nuclearization of β-catenin, the effect of Delta on Notch (Jacobsen et al., 1998) or of Neuralized on Delta (Yeh et al., 2000, 2001). Much data from experimental embryology and molecular biology are included in this model; for references see Davidson et al., Dev. Biol. 246, 162-190, 2002.
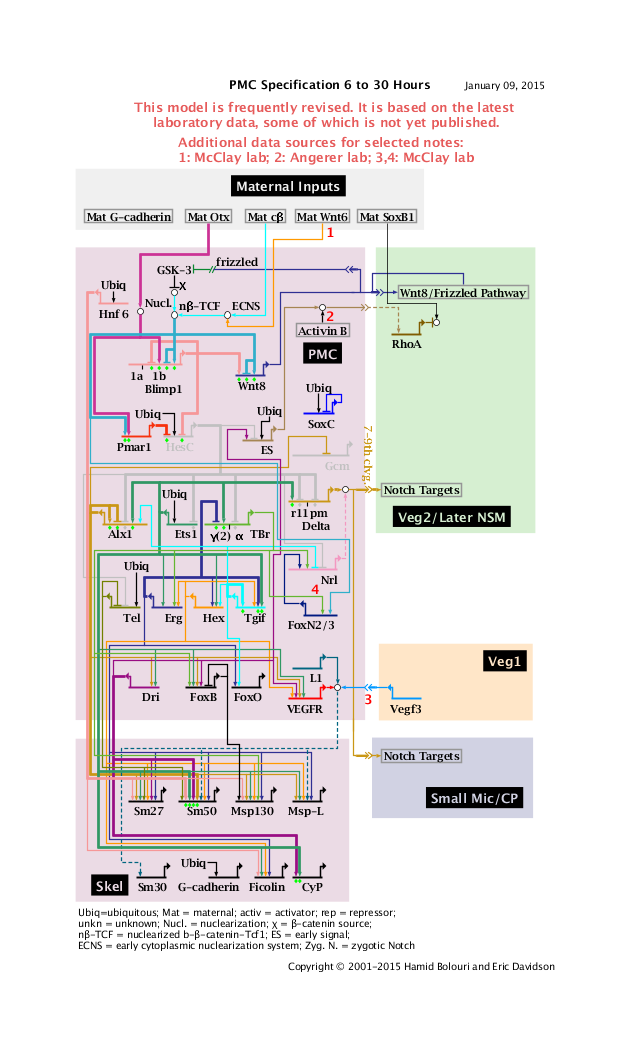
'''PMC Specification 6 to 30 Hours: View From All Nuclei''' Back to Top
Notes
1: Data from McClay Lab.
2: Data from Angerer Lab.
3: Signal and Sm30 activation only after PMC ingression. Data from McClay; extrapolated from Lytechinus variegatus.
4: TBr->FoxN2/3 data from McClay; extrapolated from Lytechinus variegatus.
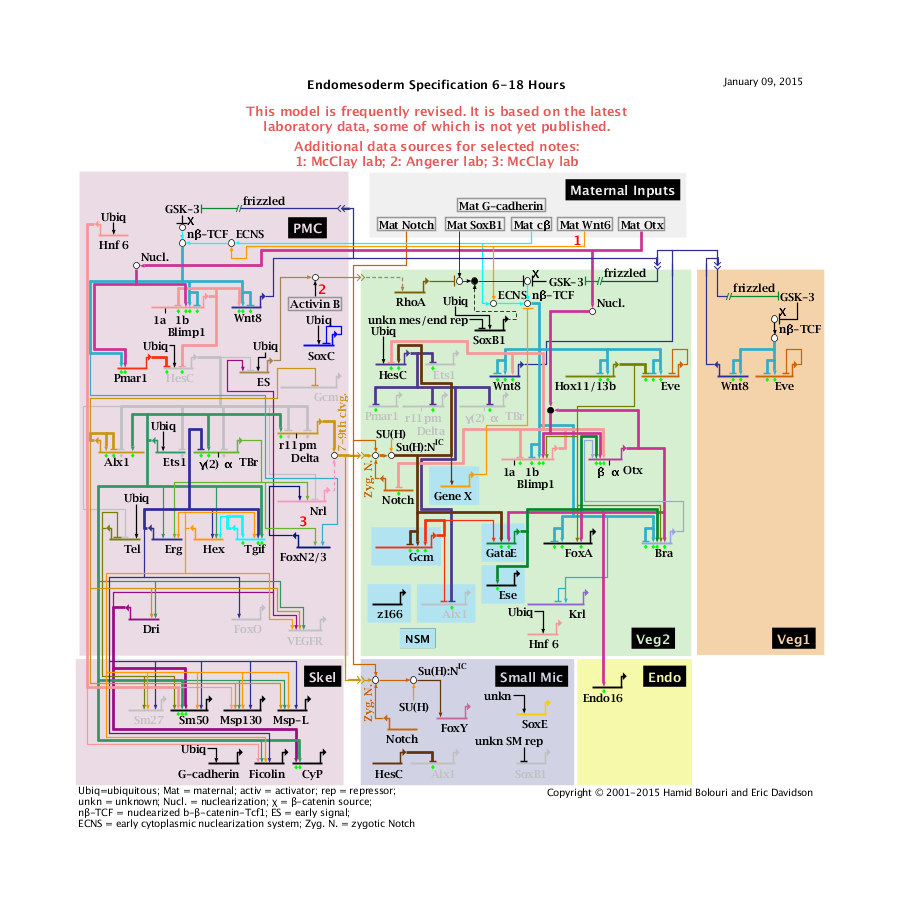
'''Endomesoderm Specification 6 to 18 Hours: View From All Nuclei''' Back to Top
Notes
1: Data from McClay Lab.
2: Data from Angerer Lab.
3: TBr->FoxN2/3 data from McClay; extrapolated from Lytechinus variegatus.
[[|]]
Endomesoderm QPCR Data Tables Back to Top
[[|]]
ECTODERM GENE NETWORK
[[|]] BioTapestry Interactive Network Viewer for Ectoderm Back to Top
The ectoderm network model can be viewed using the BioTapestry Interactive Network Viewer, which is now a web application that runs in all modern browsers. For more information about BioTapestry and running it, since the above sections About The BioTapestry Editor and BioTapestry Viewer "Quick Start" User's Guide.
Click Here to View the Ectoderm Model Using the BioTapestry Interactive Network Viewer
[[|]] Ectoderm Network Views Back to Top The following four network diagrams illustrate portions regulatory gene network for ectoderm specification. These four "View from All Nuclei" (VfA) diagrams include the Mesomere Lineages Network 6 to 17 Hours, Oral Animal Ectoderm 18 to 30 Hours, Lateral and Aboral Animal Ectoderm 18 to 30 Hours, and Veg1 Ectoderm 21 to 30 Hours.
These network views are supported by the following sources of information: (1) MASO perturbation experiments, the raw data for which are in the process of being added to the accompanying table below; (2) time course and in situ hybridization data for the regulatory genes in the network, some of which is published in the Genome Issue of Developmental Biology (v.300(1), 2006) and in the following references. Relevant additional data are from Gross et al, Development 130, 1989-1999, 2003; Duboc et al, Developmental Cell 6, 397-410, 2004; Duboc et al, ibid, 9, 147-158, 2005; Angerer et al, Development 128, 4393-4404, 2001; Bradham and McClay, ibid, 133, 21-33, 2006; Otim et al, Developmental Biology, 273, 226-243, 2004; Amore et al, ibid, 261, 55-81, 2003; Nam et al, ibid, 306, 860-869, 2007; Ben-Tabou de-Leon, S., Su, Y.-H., Lin, K.-T., Li, E., and Davidson, E. H. Developmental Biology 374, 245-254, 2013; Li, E., Cui, M., Peter, I.S., and Davidson, E. H. PNAS Online Feb 20, 2014, E906-913.
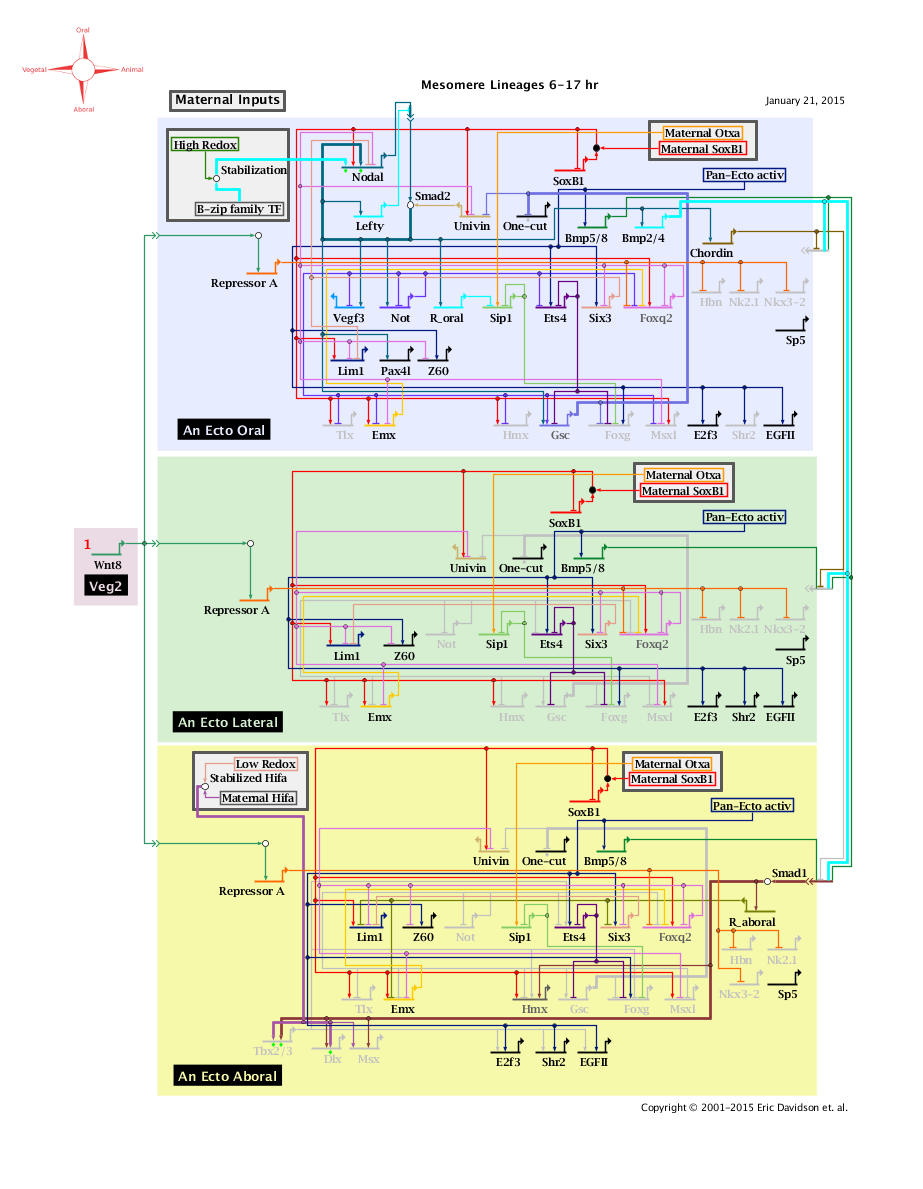
'''Mesomere Lineages Network 6 to 17 Hours: View From All Nuclei''' Back to Top
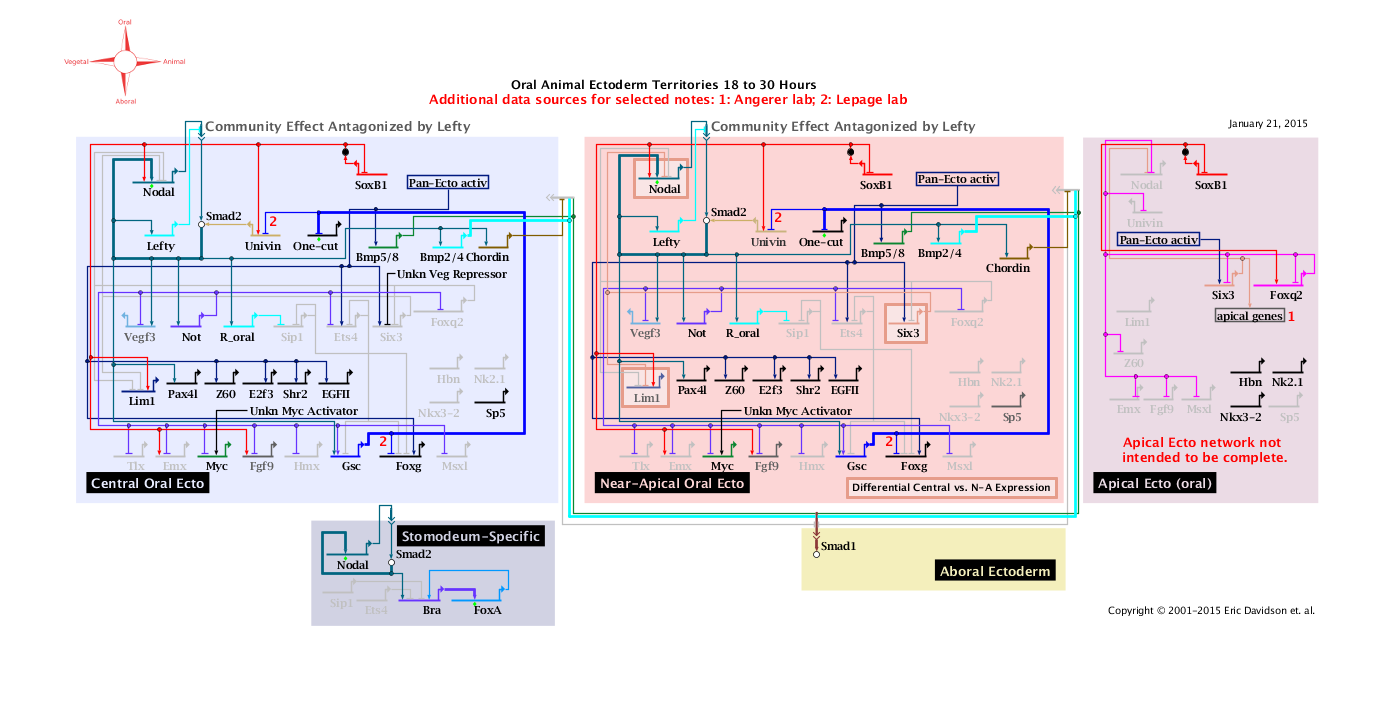
'''Oral Animal Ectoderm 18 to 30 Hours: View From All Nuclei''' Back to Top
Notes
1: Data from Angerer Lab.
2: Data from Lepage Lab.
'''Lateral and Aboral Animal Ectoderm 18 to 30 Hours: View From All Nuclei''' Back to Top
Notes
1: Data from Lepage Lab.
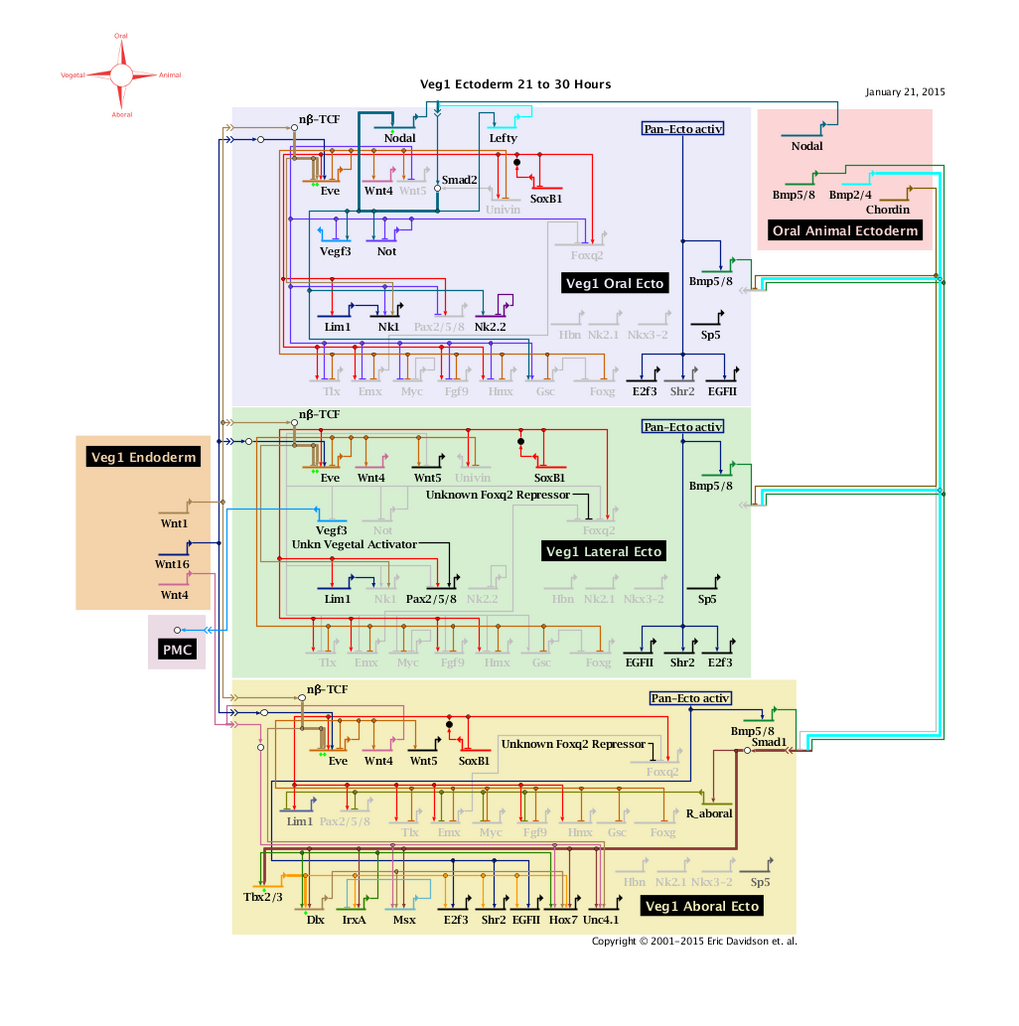
'''Veg1 Ectoderm 21 to 30 Hours: View From All Nuclei''' Back to Top
[[|]]
Ectoderm QPCR and Nanostring Data Tables Back to Top
[[|]] HIGH DENSITY TIMECOURSE DATA
High Density Timecourse Data Back to Top
Take me directly to the plotting tool.
We measured the prevalence of 172 genes - transcription factors, signaling molecules and some markers - in early sea urchin development. Most of these genes are locally restricted in their spatial expression, and contribute to the divergent regulatory states of cells in the developing embryo. Many genes in this data set are already part of the gene regulatory networks covering endomesoderm or ectoderm, others have yet to be integrated.
In order to obtain high-resolution expression profiles samples were collected at hourly intervals from fertilization to late gastrulation. Rather than rely on housekeeping genes to quantify expression level we used external standards. For this we carefully transcribed GFP RNA and RFP RNA in vitro and accurately determined its concentration. We then added a fixed number of RNA molecules to lysates of a known number of embryos. Abundance of sea urchin transcripts and standards was determined with the NanoString nCounter, an RNA counting device, and the resulting data were converted to molecules per embryo. The measured time courses agree well with, and substantially extend, prior relative abundance measurements obtained by quantitative PCR. The data are available via an interactive plotting tool for quick plotting of selected time courses. For a detailed discussion of the data please consult the paper (Materna et al., 2010).
[[|]] References Back to Top
This work is described in:
Davidson et al., A Genomic Regulatory Network for Development. Science 295 (5560): 1669-1678, 2002. (Available online.)
Bolouri, H. and Davidson, E. H. Modeling DNA sequence-based cis-regulatory gene networks. Dev. Biol. 246, 2-13, 2002.
Brown, C. T., Rust, A. G., Clarke, P. J. C., Pan, Z., Schilstra, M. J., De Buysscher, T., Griffin, G., Wold, B. J., Cameron, R. A., Davidson, E. H. and Bolouri, H. New computational approaches for analysis of cis-regulatory networks. Dev. Biol. 246, 86-102, 2002.
Ransick, A., Rast, J. P., Minokawa, T., Calestani, C. and Davidson, E. H. New early zygotic regulators of endomesoderm specification in sea urchin embryos discovered by differential array hybridization. Dev. Biol. 246, 132-147, 2002.
Yuh, C.-H., Brown, C. T., Livi, C. B., Rowen, L., Clarke, P. J. C. and Davidson, E. H. Patchy interspecific sequence similarities efficiently identify positive cis-regulatory elements in the sea urchin. Dev. Biol. 246, 148-161, 2002.
Davidson, E. H., Rast, J. P., Oliveri, P., Ransick, A., Calestani, C., Yuh, C.-H., Minokawa, T., Amore, G., Hinman, V., Arenas-Mena, C., Otim, O., Brown, C. T., Livi, C. B., Lee, P. Y., Revilla, R., Schilstra, M. J., Clarke, P. J. C., Rust, A. G., Pan, Z. J., Arnone, M. I., Rowen, L., Cameron, R. A., McClay, D. R., Hood, L. and Bolouri, H. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 246, 162-190, 2002.
Rast, J. P., Cameron, R. A., Poustka, A. J. and Davidson, E. H. brachyury target genes in the early sea urchin embryo isolated by differential macroarray screening. Dev. Biol. 246, 191-208, 2002.
Oliveri, P., Carrick, D. M. and Davidson, E. H. A regulatory gene network that directs micromere specification in the sea urchin embryo. Dev. Biol. 246, 209-228, 2002.
Davidson, E. H., McClay, D. R. and Hood, L. Regulatory gene networks and the properties of the developmental process. Proc. Natl. Acad. Sci. USA 100, 1475-1480, 2003.
Oliveri, P., McClay, D. R. and Davidson, E. H. Activation of pmar1 controls specification of micromeres in the sea urchin embryo. Dev. Biol. 258, 32-43, 2003.
Amore, G., Yavrouian, R. G., Peterson, K. J., Ransick, A., McClay, D. R. and Davidson, E. H. Spdeadringer, a sea urchin embryo gene required separately in skeletogenic and oral ectoderm gene regulatory networks. Dev. Biol. 261, 55-81, 2003.
Calestani, C., Rast, J. P. and Davidson, E. H. Isolation of mesoderm specific genes in the sea urchin embryo by differential macroarray screening. Development 130, 4587-4596, 2003.
Bolouri, H. and Davidson, E. H. Transcriptional regulatory cascades in development: Initial rates, not steady state, determine network kinetics. Proc. Natl. Acad. Sci. USA 100, 9371-9376, 2003.
Revilla-i-Domingo, R. and Davidson, E. H. Developmental gene network analysis. Int. J. Dev. Biol. 47, 695-703, 2003.
Hinman, V. F., Nguyen, A., Cameron, R. A. and Davidson, E. H. Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc. Natl. Acad. Sci. USA 100, 13356-13361, 2003.
Minokawa, T., Rast, J. P., Arenas-Mena, C., Franco, C. B. and Davidson, E. H. Expression patterns of four different regulatory genes that function during sea urchin development. Gene Expression Patterns 4, 449-456. 2004.
Yuh, C.-H., Dorman, E. R., Howard, M. L. and Davidson, E. H. An otx cis-regulatory module: A key node in the sea urchin endomesoderm gene regulatory network. Dev. Biol. 269, 536-551, 2004.
Howard, M. L. and Davidson, E. H. cis-Regulatory control circuits in development. Dev. Biol. 271, 109-118, 2004.
Otim, O., Amore, G., Minokawa, T., McClay, D. R. and Davidson, E. H. SpHnf6, a transcription factor that executes multiple functions in sea urchin embryogenesis. Dev. Biol. 273, 226-243,2004.
Oliveri, P. and Davidson, E. H. Gene regulatory network controlling embryonic specification in the sea urchin. Curr. Opin, Genet. Dev. 14, 351-360, 2004.
Hinman, V. F. and Davidson, E. H. System-level properties revealed by a gene regulatory network analysis of pregastrular specification in sea urchins. In "Gastrulation," Stern, C. D. (ed.), Cold Spring Harbor Press, Cold Spring Harbor, NY, pp. 643-652, 2004.
Revilla-i-Domingo, R., Minokawa, T. and Davidson, E. H. R11: A cis-regulatory element that controls expression of SpDelta and responds to the Pmar1 repression system. Dev. Biol. 274, 438-451, 2004.
Lee, P. Y. and Davidson, E. H. Expression of Spgatae, the Strongylocentrotus purpuratus ortholog f vertebrate GATA4/5/6 factors. Gene Exp. Patterns 5, 161-165, 2004.
Levine, M. and Davidson, E. H. Gene regulatory networks for development. Proc. Natl. Acad. Sci. USA 102, 4936-4942, 2005.
Istrail, S. and Davidson, E. H. Logic functions of the genomic cis-regulatory code. Proc. Natl. Acad. Sci. USA 102, 4954-4959, 2005.
Yuh, C.-H., Dorman, E. R. and Davidson, E. H. Brn1/2/4, the predicted midgut regulator of the endo16 gene of the sea urchin embryo. Dev. Biol. 281, 286-298, 2005.
Longabaugh, W. J. R., Davidson, E. H. and Bolouri, H. Computational representation of developmental genetic regulatory networks. Dev. Biol. 283, 1-16, 2005.
Livi, C. B. and Davidson, E. H. Expression and function of blimp1/krox, an alternatively transcribed regulatory gene of the sea urchin endomesoderm network. Dev. Biol. 293, 513-525, 2006.
Ransick, A. and Davidson, E. H. cis-Regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev. Biol. 297, 587-602, 2006.
Davidson, E. H. Special issue: The sea urchin genome. Dev. Biol. 300, 1, 2006.
Howard-Ashby, M., Materna, S. C., Brown, C. T., Tu, Q., Oliveri, P., Cameron, R. A. and Davidson, E. H. High regulatory gene use in sea urchin embryogenesis: Implications for bilaterian development and evolution. Dev. Biol. 300, 27-34, 2006.
Tu, Q., Brown, C. T., Davidson, E. H. and Oliveri, P. Sea urchin forkhead gene family: Phylogeny and embryonic expression. Dev. Biol. 300, 49-62, 2006.
Howard-Ashby, M., Materna, S. C., Brown, C. T., Chen, L., Cameron, A. and Davidson, E. H. Identification and characterization of homeobox transcription factor genes in S. purpuratus, and their expression in embryonic development. Dev. Biol. 300, 74-89, 2006.
Howard-Ashby, M., Brown, C. T., Materna, S. C., Chen., L. and Davidson, E. H. Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev. Biol. 300, 90-107, 2006.
Materna, S. C., Howard-Ashby, M., Gray, R. F. and Davidson, E. H. The C2H2 zinc finger genes of Strongylocentrotus purpuratus and their expression in embryonic development. Dev. Biol. 300, 108-120, 2006.
Oliveri, P., Walton, K. D., Davidson, E. H. and McClay, D. R. Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development 133, 4173-4181, 2006.
Davidson, E. H. The Sea Urchin Genome: Where will it lead us? Science 314, 939-940, 2006.
Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314, 941-952, 2006.
Samanta, M. P., Tongprasit, W., Istrail, S., Cameron, R. A., Tu, Q., Davidson, E. H. and Stolc, V. The transcriptome of the sea urchin embryo. Science 314, 960-962, 2006.
Ben-Tabou de-Leon, S. and Davidson, E. H. Deciphering the underlying mechanism of specification and differentiation: The sea urchin gene regulatory network. Science STKE 361, pe47, [DOI: 10.1126/stke.3612006pe47], 2006.
Livi, C. B. and Davidson, E. H. Regulation of spblimp1/krox1a, an alternatively transcribed isoform expressed in midgut and hindgut of the sea urchin gastrula. Gene Exp. Patterns 7, 1-7, 2007.
Ben-Tabou de-Leon, S. and Davidson, E. H. Gene regulation: Gene control network in development. Ann. Rev. Biophys. Biomol. Struct. 36, 191-212, 2007.
Oliveri, P. and Davidson, E. H. Built to run, not fail. Science 315, 1510-1511, 2007.
Nam, J., Su, Y.-H., Lee, P. Y., Robertson, A. J., Coffman, J. A. and Davidson, E. H. cis-Regulatory control of the nodal gene, initiator of the sea urchin oral ectoderm gene network. Dev. Biol. 306, 860-869, 2007.
Lee, P. Y., Nam, J. and Davidson, E. H. Exclusive developmental functions of gatae cis-regulatory modules in the Strongylocentrotus purpuratus embryo. Dev. Biol. 307, 434-445, 2007.
Revilla-i-Domingo, R., Oliveri, P. and Davidson, E. H. A missing link in the sea urchin embryo gene regulatory network: hesC and the double-negative specification of micromeres. Proc. Natl. Acad. Sci. USA 104, 12383-12388, 2007. (PMCID: PMC 1941478.)
Materna, S. C. and Davidson, E. H. Logic of gene regulatory networks. Curr. Opin. Biotechnol. 18, 351-354, 2007.
Smith, J., Theodoris, C. and Davidson, E. H. A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science 318, 794-797, 2007.
Smith, J., Kraemer, E., Liu, H., Theodoris, C. and Davidson, E. H. A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embryo. Dev. Biol. 313, 863-875, 2008.
Oliveri, P., Tu, Q. and Davidson, E. H. Global regulatory logic for specification of an embryonic cell lineage. Proc. Natl. Acad. Sci. USA 105, 5955-5962, 2008.
Smith, J. and Davidson, E. H. Gene regulatory network subcircuit controlling a dynamic spatial pattern of signaling in the sea urchin embryo. Proc. Natl. Acad. Sci. USA 105, 20089-20094, 2008.
Davidson, E. H. and Levine, M. Properties of developmental gene regulatory networks. Proc. Natl. Acad. Sci. USA 105, 20063-20066, 2008.
Ben-Tabou de-Leon, S. and Davidson, E. H. Modeling the dynamics of gene regulatory networks. Dev. Biol. 325, 317-328, 2009.
Davidson, E. H. Developmental biology at the systems level. Biochim. Biophys. Acta Gene Regulatory Mech. 1789, 248-249, 2009.
Longabaugh, W. J. R., Davidson, E. H. and Bolouri, H. Visualization, documentation, analysis, and communication of large scale gene regulatory networks. Biochim. Biophys. Acta Gene Regulatory Mech. 1789, 363-374, 2009.
Su, Y.-H., Li, E., Geiss, G. K., Longabaugh, W. J. R., Krämer, A. and Davidson, E. H. A perturbation model of the gene regulatory network for oral and aboral ectoderm specification in the sea urchin embryo. Dev. Biol. 329, 410-421, 2009.
Peter, I. and Davidson, E. H. Genomic control of patterning. Int. J. Dev. Biol. 53, 707-716, 2009.
Li, E. and Davidson, E. H. Building developmental gene regulatory networks. Birth Defects Res. (Part C) 87, 123-130, 2009.
Bolouri, H. and Davidson, E. H. The gene regulatory network basis of the "community effect," and analysis of a sea urchin embryo example. Dev. Biol., in press, available online.
Ben-Tabou de-Leon, S. and Davidson, E. H. Experimentally based sea urchin gene regulatory network and the causal explanation of developmental phenomenology. Wiley Interdisciplinary Reviews: Systems Biol. & Med. 1, 237-246, 2009.
Wahl, M. E., Hahn, J., Gora, K., Davidson, E. H. and Oliveri, P. The cis-regulatory system of the tbrain gene: Alternative use of multiple modules to promote skeletogenic expression in the sea urchin embryo. Dev. Biol. 335, 428-441, 2009.
Smith, J. and Davidson, E. H. Regulative recovery in the sea urchin embryo, and the stabilizing role of fail-safe gene network wiring. Proc. Natl, Acad. Sci. USA, 106, 18291-18296, 2009.
Davidson, E. H. Network design principles from the sea urchin embryo. Curr Opin. in Genetics Dev., in press, available online.
Davidson, E. H. and Erwin, D. H. Evolutionary innovation and stability in animal gene networks. J. Evol. Zool., in press.
Cameron, R. A. and Davidson, E. H. Flexibility of transcription factor target site position in conserved cis-regulatory modules. Dev. Biol., in press, available online.
Peter, I. S. and Davidson, E. H. The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev. Biol., in press, available online.
Peter, I. S. and Davidson, E. H. Modularity and design principles in the sea urchin embryo gene regulatory network. FEBS Letters, in press.